

|
Home
| Databases
| WorldLII
| Search
| Feedback
University of New South Wales Law Journal Student Series |
A CROSS-CONTINENTAL ANALYSIS OF THE MYRIAD CASES: TO WHAT EXTENT HAVE THEY REDUCED THE PATENTABILITY OF BIOTECHNOLOGY INNOVATIONS?
LEONIE OLIVIA LECLERC
The fast-paced development of biotechnology combined with its technical complexity has made it a challenging area to legislate. The responsibility has consequently been imposed upon Judges to ensure that patent law adequately protects and incentivises innovation. The High Court of Australia case D'Arcy v Myriad Genetics Inc [2015] HCA 35 and the United States Supreme Court case Association for Molecular Pathology v Myriad Genetics Inc, 569 US 576 (2013) marked a turning point for patent law in biotechnology; by declaring claims for isolated gene sequences unpatentable, the Myriad cases generated controversy across the legal and biotechnology industry. This essay comparatively analyses the degree to which the cases have reduced the patentability of biotechnology innovations by examining the judgments themselves and the aftermath of the cases across both jurisdictions. It concludes that the judgment of the Australian High Court narrowed the scope of patentable subject matter to a greater extent than the US Supreme Court: first, by its characterisation of the isolated gene sequence; second, by invalidating the patentability of cDNA; and third, by imposing policy considerations. However, the High Court decision has not made a profound impact on subsequent biotechnology cases. Interestingly, this essay finds that the current Australian legislative framework has reduced the patentability of biotechnology innovations to a significantly lesser extent than that of the United States due to its comparatively narrow interpretation.
I INTRODUCTION
Fifty years ago, it would have been unfathomable that courts across the world would be divided on whether fragments of our genetic heritage are—and should be—patentable. As of 2019, the biotechnology industry is developing faster than ever. Yet, as expected from a field that combines technology, human bodies and the law, the industry has bred tension at an ethical, political and judicial level. This essay is particularly interested in the judicial dimension, as the highest courts of the US and Australia have recently marked a turning point for patent law.
In 2013, the Supreme Court of the United States (‘Supreme Court’) ruled that claims for isolated DNA sequences were unpatentable in Association for Molecular Pathology v Myriad Genetics Inc (‘Myriad’).[1] Two years later, the Australian High Court (‘High Court’) decision in D’Arcy v Myriad Genetics Inc (‘D’Arcy’)[2] followed in the footsteps of the US, declaring claims for isolated gene sequences—as well as complementary deoxyribonucleic acid (cDNA)—unpatentable. The parallel between the US and Australian legal systems combined with the US’s ability to institute persuasive precedent on the Australian legal landscape provides the opportunity for an insightful comparative analysis.[3]
This essay compares the degree to which these cases have reduced the patentability of biotechnology innovations in their respective jurisdictions. Part II begins with an overview of Myriad Genetics Inc (‘MGI’), a brief scientific background, and the relevant legislative framework. The crux of the essay is approached by a two-step process: first, by analysing the judgments themselves in Part III, and second, by analysing the aftermath of the cases in Part IV. Part III adopts a threefold approach to conclude that the judgment of the High Court adopted a stricter patent regime than the Supreme Court: first, by its characterisation of the isolated gene sequence; second, by invalidating the patentability of cDNA; and third, by imposing policy considerations. Part IV, however, examines the aftermath of Myriad and D’Arcy. It concludes that interestingly, Australia has reduced the patentability of biotechnology innovations to a lesser extent than the US.
II BACKGROUND
A Myriad Genetics Inc & Procedural History
MGI is a self-proclaimed ‘pioneer and leader in molecular diagnostics’ founded in 1991 in Salt Lake City, Utah, US.[4] MGI has discovered numerous disease-causing genes since its founding. In 1994, MGI identified the BRCA1 and BRCA2 genes, which can be isolated from patients to determine their risk of developing hereditary breast and ovarian cancer through BRCA testing.[5] MGI successfully filed a patent claim with the United States Patent and Trademarks Office (‘USPTO’) for the BRCA1 gene in 1994, and the BRCA2 gene in 1995.[6] By patenting the genes themselves, MGI had established a monopoly over the performance of the BRCA test by 1999. This prompted the American Civil Liberties Union (‘ACLU’) to file a lawsuit against MGI in 2009, which aimed to legally challenge the validity of MGI’s patents. The case ultimately ended in the Supreme Court in 2013, where it was unanimously held that naturally occurring isolated DNA sequences were products of nature, and thus patent-ineligible.[7]
In Australia, the patent for the BRCA1 gene was filed successfully with the Australian Patent Office (‘APO’) in 1995.[8] In 2010, a claim was brought against MGI on behalf of Yvonne D’Arcy, who sought to revoke MGI’s patent over the BRCA1 gene.[9] The Full Federal Court found against D’Arcy in 2014, arguing that an isolated gene sequence sufficiently satisfies the Australian patent law tests. This decision was overturned by the High Court in 2015, which unanimously found that the isolated nucleic acid coding for the BRCA1 protein was not patent-eligible.[10]
B Scientific Background
Deoxyribonucleic acid (DNA) is a complex molecule containing genetic instructions that govern the development, growth and functions of cells.[11] A gene is a unique section of DNA; these sections are better understood as sequences of nucleotides that code for specific proteins. Genes coil to make up a chromosome. Each cell in the human body contains 23 pairs of chromosomes inside their nucleus.[12]
The BRCA1 gene is located on chromosome 13. Scientists at MGI were able to identify the BRCA1 gene by identifying the protein of interest and then isolating the gene that codes for it.[13]
Transcription is the natural process where genetic information encoded in DNA is copied into Messenger Ribonucleic acid (mRNA). Through translation, the genetic information encoded in mRNA is then used to assemble amino acids to make proteins. Transcription can be artificially reversed through reverse transcription to create cDNA.[14]
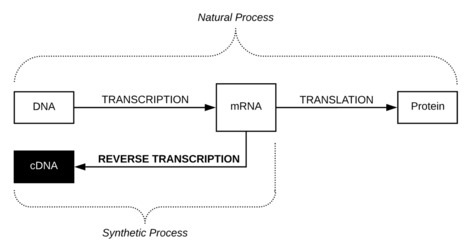
Figure 1: Overview of cDNA process
C Statutory & Legislative Framework
The US and Australian legislative instruments do not explicitly define the sorts of inventions or discoveries that fall outside the scope of patentability. It has thus ‘been in province of the courts to establish limits for subject matter that may not be granted patent protection.’[15]
1 United States
Article I § 8 Clause 8 of the United States Constitution is the Patent and Copyright Clause.[16] It stipulates that:
‘Congress shall have the power to promote progress of science and useful arts by securing for limited times to authors and inventors the exclusive rights to their respective writings and to their discoveries.’
This clause is the foundation for the development of patent law in the US. The relevant legislation referred to in Myriad is § 101 under Title 35 of the United States Code (USC),[17] which defines patentable subject matter as follows:
‘Whoever invents or discovers any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof, may obtain a patent therefore, subject to the conditions and requirements of this title.’
35 USC § 101 has developed over the years to require the invention be novel, non-obvious, and useful.[18] The ‘product of nature’ doctrine considers ‘laws of nature, abstract ideas and physical phenomena...’ patent-ineligible.[19] It requires a claim to be ‘markedly different’ from its naturally-occurring counterpart.[20]
2 Australia
Patent law in Australia is governed by the Patents Act 1990.[21] Section 18(1) of the Act requires an invention to be ‘a manner of manufacture within the meaning of s 6 of the Statute of Monopolies’, ‘novel’, ‘useful’ and ‘involve an inventive step’. Subsection (2) stipulates that ‘human beings, and biological process for their generation, are not patentable invention.’
In analysing the meaning of ‘manner of manufacture’, the High Court in National Research Development Corporation v Commissioner of Patents (‘NRCD’) emphasised that restricting this concept would be ‘unsound to the point of folly’ in light of scientific advances.[22] NRCD thus characterised this concept by examining whether the relevant claim was an ‘artificially created state of affairs’ of which the ‘significance...is economic.’[23]
In 2013, the High Court affirmed this test in Apotex Pty Ltd v Sanofi-Aventis Australia Pty Ltd,[24] finding that ‘if a process...results in a new and useful effect, so that the new result is an artificially created state of affairs providing economic utility, it may be considered a manner of new manufacture’.[25]
III THE JUDGMENTS
A Characterising the Isolated Gene Sequence
1 ‘Information’
The characterisation of an isolated gene sequence was central to the Supreme Court and High Court’s decision to invalidate their patentability. The courts considered whether isolated gene sequences should be analysed from a structural, chemical or carrier of information standpoint.[26]
Both courts vehemently rejected the characterisation of the isolated gene sequence by its chemical composition. The Supreme Court overturned Judge Lourie’s argument raised in the Federal Circuit that the chemical difference between the isolated gene and the same gene inside the human genome was sufficient to not fall in the product of nature exception.[27] The Supreme Court argued that severing the chemical bonds ‘did not create a non-naturally occurring molecule’ because the claims are ‘not expressed in terms of chemical composition, nor do they rely in any way on the chemical changes that result from the isolation of a particular section of DNA.’[28] The judges further argued that a ‘would-be infringer could...isolate a DNA sequence...[and add] one additional nucleotide pair. Such a molecule would not be chemically identical to the [original] molecule.’[29] The Supreme Court stated that the claims should instead ‘focus on the genetic information encoded in the BRCA1 and BRCA2 genes.’[30] In applying the product of nature doctrine, the Supreme Court reasoned that DNA’s information sequences and processes occur naturally within cells, and should thereby be patent-ineligible.[31]
Comparably, the High Court rejected the Full Federal Court’s argument that the gene’s difference in chemical composition sufficiently fulfilled the ‘artificial state of affairs’ test in NRCD.[32] Their Honours criticised the characterisation of the claims through their chemical composition on the basis that it ‘elevates form over substance’.[33] Instead, their Honours considered the claims more appropriately characterised as ‘information’ on the basis that the substance of the claims is ‘information embodied in arrangements of nucleotides.’[34] The High Court consequently held the claims unpatentable because ‘the information stored in the sequence of nucleotides...is the same information as that contained in the DNA of the person from which the nucleic acid was isolated.’[35]
2 Analysis & Criticism
The Myriad and D’Arcy judgments’ characterisation of the claims are rife with inconsistencies and questionable science applications. First, the Supreme Court’s hypothetical that one could add a nucleotide pair to an isolated gene sequence (thereby making it ‘chemically different’) demonstrates a lack of scientific understanding; an additional nucleotide pair may change its function and potentially result in disease-causing mutations.[36] Interestingly, this is something the Supreme Court itself discusses in Myriad, [37] highlighting internal inconsistencies within the judgment.
Sherman criticises the courts’ characterisation of the claims as ‘information’, arguing that ‘while genes do embody information...it is not the information per se that is important, so much as the function of genes to shape human development.’[38] Yet as stated by Schwartz, ‘the semantic attraction of defining DNA simply as information storage seems to have provided irresistible to a generalist court with little scientific expertise.’[39] The result of the scientific inaccuracies threaded within the characterisation of the claims undoubtedly creates uncertainty within the jurisdictions’ legislative landscapes.
A significant distinction between Myriad and D’Arcy is the High Court’s reformulation of the NRCD test, which has underpinned Australian patent law for decades. NRCD criticised and rejected the US’s product of nature doctrine on the basis that ‘the distinction between discovery and invention is not precise enough to be other than misleading’.[40] However, by characterising the claims as ‘information’, the High Court arguably implemented into Australian patent law a de facto ‘product of nature’ doctrine. This represents a significant departure from NRCD.
The High Court defined ‘information’ as ‘that which inheres in or is represented by a particular arrangement, sequence, or set, that may be stored in, transferred by, and responded to by inanimate things.’[41] Their Honours’ universal definition of ‘information’ makes it applicable to all molecules and every aspect of nature; this arguably renders it broader than the Supreme Court’s characterisation of the claims as ‘genetic information’ encoded within the genes.[42] This indicates the creation of a stricter patent regime in Australia. Lawson argues that the High Court’s ‘formulation is of limited value and fails to provide any guidance about the event horizon between matter and information.’[43] This demonstrates the ambiguity and uncertainty arising by their Honours’ broad informational characterisation. However, it is important to mention that the High Court did not specifically mention biological material other than isolated naturally occurring genes in D’Arcy. This may imply that despite the High Court’s broad characterisation of ‘information’, their Honours did not intend to limit the patentability of other biological material. Shoebridge argues that it ‘seems clear that [D’Arcy] cannot be determinative in relation to excluding from patentability isolated biological material other than isolated naturally-occurring nucleic acid sequences...’[44] In any case, the contention raised by the High Court’s ambiguous characterisation of ‘information’ strongly contributes to the uncertainty created by D’Arcy.
B The Patentability of cDNA
The Supreme Court and High Court both discuss cDNA in their judgments, yet come to starkly different conclusions; whilst the Supreme Court held cDNA patentable,[45] the High Court did not.[46] The High Court reasoned that cDNA ‘replicates a naturally-occurring sequences of exons’ which means that information is ‘an essential element of [cDNA’s] invention’.[47] This highlights the High Court’s stricter patent regime, and demonstrates how their Honours’ ‘information’ characterisation was more broadly applied.
Examining this distinction is interesting considering both courts characterised the gene sequence as ‘information’. This appears to highlight the High Court’s recognition of the internal inconsistencies within Myriad’s judgment, whereby the judges argued that cDNA ‘contained [the] same protein-coding information found in segments of natural DNA’ but ‘differed from natural DNA in that the non-coding regions had been removed.’[48] Stern argues that this distinction based on ‘whether useless introns are edited out in a known and conventional manner is just artificial and irrational.’[49] Orford highlights that the ‘court appears to have concluded that isolated DNA is naturally-occurring in the informational sense and not patent-eligible,’ while simultaneously ‘finding cDNA patent-eligible on the ground that it does naturally occur in the molecular sense.’[50] This is despite the informational equivalence between DNA and cDNA, and the court’s rejection to characterise the claims through their chemical composition. Further, Stern argues that the court’s reasoning is ‘quite at odds with the case law on products of nature, because the court’s logic is simply that introns-free cDNA is not identically disclosed in natural products, rather than that cDNA is qualitatively different.’[51] It goes to show the uncertainty Myriad has created borne from technical and scientific inconsistencies.
C Policy Considerations
The High Court and Supreme Court’s application of policy considerations played a significant role in rendering isolated gene sequences unpatentable. However, the High Court’s reformulation of NRDC through the introduction of a multi-factorial approach indicates that D’Arcy created a stricter patent regime than Myriad.
1 Australia’s Multi-factorial Approach
The High Court adopted a high incorporation of policy considerations in deciding to invalidate the patentability of isolated gene sequences and cDNA. In fact, Bartlett goes so far to argue that in Australia, policy considerations played a larger role in the decision than the construction of the gene sequence.[52] The High Court expanded the classic NRCD test requiring an invention to be an artificially-created state of affairs in a field of economic endeavour by including a consideration of policy factors.[53] Their Honours justified this expansion on the basis that isolated gene sequences formed part of a new class of claims,[54] and that NRCD did not explicitly ‘preclude consideration of policy factors’.[55]
Their Honours’ multi-factorial approach includes whether patentability would: be consistent with the purpose of the act; enhance or detract from coherence of the law; fit within Australia’s international obligations; and, involve making law which should be done by the legislature.[56] This multi-factorial approach was incorporated into the Australian Patent Office (‘APO’) guidelines, whereby these factors must be taken into consideration when assessing the validity of a new class of claims.[57] D’Arcy’s rearticulation of NRCD thus introduced a stricter patent regime than Myriad by imposing upon decision-makers a consideration to take into account a range of factors external to the legal tests. Criticising the High Court’s rearticulation of NRCD, Bartlett argues that the ‘primary concern evident in the initial commentary on this purposive and consequentialist plurality reasoning is that it has introduced further uncertainty into the subject matter patentability test.’[58]
2 Application of Policy Considerations
Among the Supreme Court and High Court’s application of policy considerations, both justified excluding isolated gene sequences from patentability by raising the importance of maintaining the balance between encouraging and impeding innovation. In assessing how validating the patentability of isolate gene sequences could affect future scientific research, the High Court stated it would raise ‘the risk of a chilling effect upon legitimate innovative activity outside the formal boundaries of the monopoly.’[59] In the US, the Supreme Court stated that ‘there would be considerable danger that the grant of patents would tie up the use of basic tools of scientific and technological work and thereby inhibit future innovation premised upon them.’[60]
Both courts based this policy consideration on an incorrect assumption. In the US, Wales and Cartier state that there is ‘no evidence that a company or university has sued a scientist for performing purely academic research.’[61] In Australia, the Centre for International Economics prepared an independent report for IP Australia prior to D’Arcy.[62] It stipulated that patents play a significant role in ‘incentivising innovation and the public-private partnerships that are required to bring new human gene based medicines and diagnostics to market.’[63] Wales and Cartier further state that ‘the filing of at least one patent application has been shown to increase the chance of obtaining [Venture Capital] funding by 97%’.[64] Consequently, reducing patent protections in biotechnology ‘has potential to undermine investor confidence,’[65] thus, reducing incentive and resources for innovation. It appears that restricting patentability of biological subject matter creates the very effect the courts sought to avoid. Other policy factors were considered also, but the focus on this consideration specifically provides an example of how both courts (arguably improperly) applied policy considerations to reduce the patentability of biotechnology innovations.
3 Policy Considerations: Analysis & Criticism
In introducing a multi-factorial approach, their Honours implemented ‘soft law’ as a regulatory tool. As indicated by Hunter’s research, the regulation of new technologies attracts the ‘justice argument’ to a much higher degree than social or political issues.[66] This is clearly the case in Myriad and D’Arcy; despite the contention associated with judges making policy considerations,[67] the invention of isolating gene sequences strongly attracted courts to do so in regulating their patentability. However, the combination of a traditionally formulated legal system and highly developed technology is susceptible to difficulties. Schwartz argues that ‘Myriad relies upon its scientific findings as vital support for its holdings and the policies they represent’ yet, ‘exhibits disconnect between these holdings and supporting rationales.’[68] His research highlights ‘how supporting policy rationales expressed imperfectly in specialist biotech language creates uncertainty in the world of biotech patenting.’[69] He refers to the court’s ‘inelegantly constructed signals’, whereby ‘Myriad thereby provides confused guidance to lower courts and to the USPTO on how to interpret its ‘bright line’ holdings’.[70] This highlights how the courts’ application of policy consideration instigated uncertainty into both jurisdictions’ patent landscapes.
IV AFTERMATH OF JUDGMENTS
A The Interpretation of Myriad and D’Arcy
Examining how guidelines were amended and how ensuing cases were decided following Myriad and D’Arcy indicates that whilst the High Court judgment adopted a stricter patent regime, it was interpreted to a significantly narrower extent than the Supreme Court judgment in Myriad.
1 USPTO & APO Guidelines
The USPTO and APO are responsible for examining patent applications and validating thousands of patent claims a year.[71]
(a) USPTO: Analysis
In assessing patent eligibility pursuant to 35 USC § 101, the USPTO guidelines provide the ‘markedly different characteristics analysis’, which stipulates that ‘if...a nature-based product limitation does not exhibit markedly different characteristics [to its naturally occurring counterpart], then that limitation is a product of nature exception.’[72] This formulation thus extends beyond biotechnology inventions; Wales and Cartier argue that they also invalidate ‘claims to small-molecule therapeutics that are structurally identical to natural protects as well.’[73] They consequently argue that the USPTO guidelines’ broad reading of Myriad ‘extends the analysis [of the Supreme Court] far beyond the actual holdings [in Myriad and Mayo] and greatly decreases the scope of patentable subject matter in the United States.’[74]
(b) APO: Analysis
Following the D’Arcy decision, the APO amended their examination guidelines in the Manual of Practice and Procedure (‘the examination manual’) to declare isolated naturally-occurring genes unpatentable.[75] The examination manual also renders artificially created genetic information unpatentable if it embodies the same ‘information’ as the ‘counterpart in the genome of an organism, plant or animation’.[76] However, the examination manual explicitly clarifies that D’Arcy ‘does not set out a general rule that isolated natural products or their derivatives are excluded from patentability.’[77] This indicates that the APO has not endorsed D’Arcy as a blanket rule to restrict the patentability of such biological material. Bartlett analyses how the examinations have changed since D’Arcy, and argues that they remain narrow in scope today. This highlights that the APO has interpreted D’Arcy to a narrower extent that the USPTO. As Bartlett argues, ‘Australia’s approach starkly contrasted with the [USPTO], which broadly interpreted [Myriad].’ He reasons that ‘this disparity may be attributed to the difference between the ratios in [D’Arcy] and [Myriad].’
(c) APO & USPTO and the Myriad cases
Analysing how the USPTO and APO have interpreted the respective decisions of Myriad and D’Arcy sheds light on their practical application upon their jurisdictions’ patent law landscapes. Whilst the USPTO guidelines in the US are prospective in nature, those in Australia are demonstrably not. This is further dissected by an examination of subsequent case law.
2 Aftermath: United States
On the day the Supreme Court handed down Myriad, a number of laboratories announced that they were going to provide BRCA testing.[78] Among those, Ambry Genetics Corporation (‘Ambry Genetics’) offered to provide BRCA testing for USD$2,280 (compared to MGI’s USD$4,040).[79] This undermined MGI’s monopoly on the service, so they sought a preliminary injunction against Ambry Genetics in University of Utah Research Foundation, et al v Ambry Genetics Corporation (‘Ambry’).[80] Ambry fundamentally entrenched the Myriad decision of the Supreme Court, as it denied the injunction on the basis that MGI were ‘unable to establish that they are likely to succeed on the merits of the claims’ nor ‘that the equitable factors support issuance of the requested injunction.’[81] Denying the injunction not only allowed for Ambry Genetics to continue providing BRCA testing, but sent a signal to laboratories across the US that MGI had lost its monopoly on the service.[82] This highlights the direct application of the Supreme Court’s decision on subsequent cases; as argued by Gambini, Ambry is ‘directly related to the Supreme Court’s ruling in Myriad,’[83] and ‘[Myriad] show[s] that, at present, US courts are less willing to back the long-standing practice of granting patents on DNA sequence’.[84]
In 2014, In Roslin institute (Edinburgh) applied Myriad in holding that animals produced by asexual reproduction (famously including Dolly the sheep) were unpatentable products on the basis that cloned animals were not ‘markedly different’ from their natural counterpart.[85] This further affirmed Myriad, but has been criticised for overruling Diamond v Chakrabarty (which held that the Chakrabarty bacterium was patentable).[86]
Aria Diagnostic v Sequenom was the first case to cite Myriad in holding diagnostic method claims unpatentable.[87] Aria Diagnostics’ tests for prenatal testing was considered not inventive on the basis of Myriad’s dicta regarding an ‘innovative method of manipulating genes’.[88]
Nevertheless, it has been argued that the impact of Myriad should not be over-stated on the basis that there remain numerous ways in which patent attorneys can creatively circumvent the current legislative scheme to protect biotechnology inventions.[89] Whilst this may be the case for some, a 2016 study found that there has been an ‘overall decrease in isolated gene patents’, and that ‘the combination and joint-effect of Myriad, Mayo, and Alice make it substantially more challenging to transform ineligible claims directed to isolated genes, natural products, laws of nature, or abstract ideas into subject-matter eligible claims.’[90] This highlights Myriad’s practical impact in reducing the patentability of biological material, even with the existence of skilled lawyers. As highlighted by Robert Fischer, ‘in a recent series of cases, the Court has pretty consistently ruled in ways that narrow the scope of eligible subject.’[91]
3 Aftermath: Australia
Subsequent Australian case law on the patentability of biological material highlights the narrow interpretation of the High Court’s decision in D’Arcy. In 2016, Re Arrowhead Research Corporation (‘Arrowhead’) held claims for iRNA (double stranded RNA) patentable.[92] Delegate Calanni held that the substance of the invention should be characterised as a ‘pharmaceutical composition’ instead of ‘genetic information.’ She argued that the molecules ‘are more than a ‘step along the way to an artificially created state of affairs of economic significance’ as described in [D’Arcy] at 85.’[93] This ratio reflects the High Court’s decision in Apoex v Sanofi in 2013,[94] which applied NRDC and stipulated that ‘iRNA compositions are economically significant products that meet the requirements of manner of manufacture.’[95] Delegate Calanni’s reference to the High Court’s position on the patentability of genetic information prior D’Arcy highlights the APO’s circumvention of D’Arcy, and hesitation to read it broadly. Shoebridge argues that the decision in Arrowhead ‘clarifies and confirms the limited impact of the [D’Arcy] decision and leaves the door open for patents covering other artificially-created genes’, even cDNA.[96]
Similar cases demonstrate the narrow reading of D’Arcy. In Cargill Incorporated v Dow AgroSciences LLC (‘Cargill’),[97] a codon-optimised sequence for a Δ-9 desaturase gene was held patentable despite it coding for a naturally-occurring protein due to its economic utility.[98] Cargill indicates that D’Arcy only applies to isolated naturally-occurring genes; naturally-occurring proteins and micro-organisms remain patentable. This can be contrasted with Myriad in the US, which denied the patent eligibility for all isolated naturally-occurring materials, indicating the US’s broad interpretation of Myriad.[99] Further, Commonwealth Scientific and Industrial research organisation v BASF Plant Sciences GmBH,[100] held that genetic information was patentable because it fell within the meaning of ‘manner of manufacture’ due to the human intervention required for its creation.[101] The fact that genetic information has been held patentable since D’Arcy further demonstrates its narrow interpretation in Australia.
4 The interpretation of Myriad and D’Arcy: Conclusion
Overall, it appears that D’Arcy was interpreted to only affect the patentability of naturally occurring isolated sequences, whilst Myriad was interpreted to apply to naturally occurring biological material more broadly. This demonstrates that in practice, the High Court’s decision in D’Arcy has been interpreted to a significantly narrower extent than the Supreme Court’s decision in Myriad. This suggests that the US has reduced the patentability of biotechnology innovations to a larger extent than Australia.
B The Shared Issue of Uncertainty
Despite the substantial differences between Myriad and D’Arcy, the issue binding them is the heightened uncertainty they have introduced for the patentability of biological material. Klusty and Weinmeyer disagree, arguing that Myriad ‘helped to delineate the boundaries between those [naturally-occurring] products...and those that are the result of human innovation and creation. This should give those working on the cutting edge of genetics and medicine a clearer idea of which ideas can [be patented].’[102] Respectfully, this is far from what has occurred. The UPSTO’s decision to broaden the scope of Myriad has severely reduced the clarity of what falls within the scope of patent-eligibility;[103] as Schwartz argues, ‘it is still not entirely clear how much modification is required to render a molecule sufficiently distinct from naturally-occurring counterparts.’[104] Further, how can certainty be achieved when the judgment itself is scattered with technical and scientific inconsistencies? As stated by Burk, Myriad has left open many questions,[105] and is ‘far from illuminating’.[106]
Meanwhile, the High Court’s rearticulation of NRCD and the APO’s consistent circumvention of D’Arcy renders it difficult to predict whether certain biological products are patent-eligible. Uncertainty has also arisen as a result of inconsistency. Shoebridge argues that Cargill is inconsistent with D’Arcy’s decision to render cDNA unpatentable; whilst D’Arcy rejected claims relating to cDNA’s commercial advantages, the patent in Cargill was validated on the basis of its economic utility.[107]
The uncertainty Myriad and D’Arcy have introduced is problematic. Sufficient certainty is important to attract investment in research and development, and avoid costs associated with patent infringement.[108] The latter point was highlighted by Mac Cowell (founder of DIYBio), who stated that ‘when it becomes a problem is when the patent office grants patents that are so [expletive] wide, you don’t even know if you’re violating them.’[109] Of course, retaining a degree of flexibility is important in light of technological development;[110] as argued by Surden, ‘a possibly beneficial artefact of an uncertainly scoped claim is that it permits courts ex post flexibility to correct for under-inclusion and under-compensation.’[111] However, Surden elaborates that certainty and flexibility are not mutually exclusive, and that it is possible to uphold these concepts without creating Myriad and D’Arcy’s disarrayed patent landscapes.[112]
The essay began with the proposition that fifty years ago, it would have been unfathomable that courts across the world would be divided on whether fragments of our genetic heritage are and should be patentable. Such rapid scientific advancement highlights the issues posed by technological development in the legal sphere; how can the law—and should the law—regulate such a fast-developing climate? Applying Brownsword’s scholarship on regulatory disconnection, the revolutionary invention of isolating genetic sequences entered a regulatory void, as this phenomenon would have been beyond contemplation when patent law was developed.[113] As D’Arcy and Myriad demonstrates, this invention has consequently rendered terms such as ‘product of nature’ and ‘manner of manufacture’—which are at the core of patentability tests—ambiguous. The creation of ambiguity demonstrates how technological development potentially generates uncertainty and highlights the requirement for courts and legislators alike clarify the legal position on such issues with certainty and flexibility. Analysing the overwhelming confusion and inconsistencies introduced by D’Arcy and Myriad demonstrates that the Supreme Court and High Court did not achieve this. Perhaps it is for the legislature to use legislative instruments as a regulatory tool rather than leave such technically-complex decisions to judges who do not possess the scientific expertise.[114] Overall, D’Arcy and Myriad’s interpretation and practical application suggests that the US has reduced the patentability of biotechnology innovation to a larger extent than in Australia. However, the heightened uncertainty both decisions have introduced renders it difficult to measure the extent to which they have reduced the patentability of biotechnology innovations in their jurisdictions.
V CONCLUSION
Comparatively analysing the High Court and Supreme Court judgments in D’Arcy and Myriad demonstrates that D’Arcy adopted a stricter patent regime than Myriad. However, the US has reduced the patentability of biotechnology innovations to a larger extent due to its broader interpretation of Myriad. Irrespective of the differences in how the cases have been interpreted, they both highlight how technological development has complexified and introduced uncertainty into their respective jurisdictions.
Further, the practical impact of Myriad and D’Arcy on research and development in biotechnology has only been tested in a few cases; it is consequently too early to discern their long-term ramifications on innovation.
BIBLIOGRAPHY
A Articles/Books/Reports
Aboy, Mateo, Johnathon Liddicoat, Kathleen Liddell, Matthew Jordan and Cristina Crespo, ‘After Myriad, what makes a gene patent claim ‘markedly different’ from nature?’ (2017) 35(9) Nature Biotechnology 820
Aboy, Mateo, Kathy Liddell, Johnathon Liddicoat and Cristina Crespo, ‘Myriad’s impact on gene patents’ (2016) 34 National Biotechnology 1119
Bartlett, William, ‘D’Arcy v Myriad Genetics Inc [2015] HCA 35: The Plurality’s new factorial approach to patentability rearticulates the question asked in NRDC’ (2015) 24(1) Journal of Law, Information and Science 120
Bennett Moses, Lyria, ‘How to Think about Law, Regulation and Technology: Problems with ‘Technology as a Regulatory Target’ (2013) 5(1) Law, Innovation and Technology 1
Brownsword, Roger and Karen Yeung (eds), Regulating Technologies: Legal Futures, Regulatory Frames and Technological Fixes (Hart Publishing, 2008)
Burk, Dan, ‘Are Human Genes Patentable?’ (2013) 44(7) International Review of Intellectual Property and Competition 747
Burk, Dan, ‘Dolly and Alice’ (2015) 2(3) Journal of Law and the Biosciences 606
Chandrasekharan, Subhashini, Amy McGuire and Ignatia Van den Veyver, ‘Do recent US Supreme Court rulings on patenting of genes and genetic diagnostics affect the practice of genetic screening and diagnosis in prenatal and reproductive care?’ (2014) 34(10) Prenatal Diagnosis 921
Cook-Deegan, Robert and Annie Niehaus, ‘After Myriad: Genetic Testing in the Wake of Recent Supreme Court Decisions about Gene Patents’ (2014) 2(4) Current Genetic medicine Reports 223
Dent, Chris, ‘The Possibilities of a Regulatory Approach to Answer the Question: Should Genetic Inventions be Patentable?’ (2012) 22(1) Journal of Law, Information and Science 16
French, Robert, ‘United States Influence on the Australian Legal System’ [2018] UWALawRw 2; (2018) 43(1) University of Western Australia Law Review 11
Gambini, Emanuela, ‘The Product of Nature Doctrine in the Myriad Saga II’ (2013) 4(3) European Journal of Risk Regulation 409
Gambini, Emanuela, ‘In the Aftermath of the ‘Myriad Case’—Myriad is Denied Preliminary Injunction Against Ambry Genetics’ (2014) 5(3) European Journal of Risk Regulation 407
Gambini, Emanuela, ‘In the Aftermath of D’Arcy v. Myriad Genetics Inc: Patenting Isolated Nucleic Acids in Australia’ (2016) 7(2) Journal of Risk Regulation 451
Häussler, Carolin, Dietmar Harhoff and Elisabeth Müller, ‘To be financed or not: the role of patents for venture capital financing’ (Discussion Paper, Centre of Economic Policy Research, 28 February 2008)
Hunter, David, ‘How to object to radically new technologies on the basis of justice: the case of synthetic biology.’ (2013) 27(8) Bioethics 426
Klusty, Tobin and Richard Weinmeyer, ‘Supreme Court to Myriad Genetics: Synthetic DNA is Patentable but Isolated Genes Are Not’ (2015) 17(9) AMA Journal of Ethics 849
Lawson, Charles, ‘Patenting Nucleic Acid Sequences: More Ambiguity from the High Court?’ (2018) 25 Journal of Law and Medicine 741
Mason, Anthony, ‘Legislative and Judicial Law-Making: Can We Locate an Identifiable Boundary?’ [2003] AdelLawRw 4; (2003) 24(1) Adelaide Law Review 15
Odell-West, Amanda, ‘Gene-uinely patentable? The distinction in biotechnology between discovery and invention in US and EU patent law’ (2011) 3 Intellectual Property Quarterly 304
Orford, Jack, ‘Association for Molecular Pathology v Myriad Genetics Inc: Are Genes Information or Molecules?’ [2014] SydLawRw 24; (2014) 36(3) Sydney Law Review 557
Schwartz, Robert, ‘Life after Myriad: the uncertain future of patenting biomedical innovation and personalised medicine in an international context’ (2015) 3 Intellectual Property Quarterly 189
Sherman, Brad, ‘D’Arcy v Myriad Genetics Inc: Patenting Genes in Australia’ [2015] SydLawRw 6; (2015) 37(1) Sydney Law Review 135
Stern, Richard, ‘Association for Molecular Pathology v Myriad Genetics: sieving the gene pool’ (2013) 35(11) European Intellectual Property Review 685
Surden, Harry, ‘Efficient uncertainty in Patent Interpretation’ (2011) 68 Washington & Lee Law Review 1737
The Centre for International Economics, Economic Analysis of the Impact of Isolated Human Gene Patents (Final Report, May 2013)
Wales, Michele and Eddie Cartier, ‘The Impact of Myriad on the Future Development and Commercialization of DNA-Based Therapies and Diagnostics’ (2015) 5(12) Cold Spring Harbor Perspectives in Medicine 1
B Cases
Alice Corp v CLS Bank International, 573 US 208 (2014)
Aria Diagnostic Inc v Sequenom Inc, 726 F 3d 1296 (Fed Cir, 2013)
Arrowhead Research Corporation [2016] APO 70
Association for Molecular Pathology v Myriad Genetics Inc, 569 US 576 (2013)
Association for Molecular Pathology v US Patent and Trademark Office, 653 F 3d 1329 (Fed Cir, 2011)
Apotex Pty Ltd v Sanofi-Aventis Australia Pty Ltd [2013] HCA 50
Cargill Incorporated v Dow AgroSciences LLC [2016] APO 43
Commonwealth Scientific and Industrial Research Organisation v BASF Plant Sciences GmBH [2016] APO 83
D’Arcy v Myriad Genetics Inc [2015] HCA 35
D’Arcy v Myriad Genetics Inc [2014] FCAFC 115
Diamond v Chakrabarty[1980] USSC 119; , 447 US 303 (1980)
In Re Roslin institute (Edinburgh), 750 F 3d 1333 (Fed Cir, 2014)
Mayo Collaborative Services v Prometheus Laboratories Inc, 566 US 66 (2012)
University of Utah Research Foundation et al v Ambry Genetics Corporation, 774 F 3d 755 (Fed Cir, 2014)
National Research Development Corporation v Commissioner of Patents [1959] HCA 67
C Legislation
Patents Act 1990 (Cth)
Statute of Monopolies (1624)
United States Code
United States Constitution
D Other
Amgen, ‘Identification and Isolation of the Gene of Interest’ (YouTube, 15 August 2016, 00:00:36 – 00:00:45) <https://www.youtube.com/watch?time_continue=38&v=aYhf-FXpoYs>
Australian Patent Office, ‘2.9.2.6 Nucleic Acids and Genetic Information’ Manual of Practice and Procedure Manual, <http://manuals.ipaustralia.gov.au/patents/Patent_Examiners_Manual.htm#customer_service/Customer_Service_Charter_Timeliness_Guidelines.htm>
‘cDNA (Complementary DNA)’ HumanGenes.Org (Web Page) <http://humangenes.org/cdna-complementary-dna>
Ferguson, Cat, ‘The Future of Biohacking in The Age of Patent Trolls’ The Awl (12 December 2013) <https://www.theawl.com/2013/12/the-future-of-biohacking-in-the-age-of-patent-trolls/>
Fischer, Robert, ‘US Case Analysis: Myriad at the Supreme Court’ Managing Intellectual Property (Web Page, 1 September 2013) <https://www.managingip.com/Article/3261961/US-case-analysis-Myriad-at-the-Supreme-Court.html?ArticleId=3261961>
Harrison, Dan, ‘Genetic patents: Grandmother Yvonne D’Arcy takes on global giant Myriad Genetics’ The Sydney Morning Herald (online, 16 June 2015) <https://www.smh.com.au/politics/federal/genetic-patents-grandmother-yvonne-darcy-takes-on-global-giant-myriad-genetics-20150616-ghphvj.html>
‘History’, Myriad (Web Page) <https://myriad.com/about-myriad/inside-myriad/history/>
‘How many chromosomes do people have?’ U.S. National Library of Medicine (Web Page, 2 April 2019) <https://ghr.nlm.nih.gov/primer/basics/howmanychromosomes>
Shoebridge, Grant, ‘Should the Australian Patent Office be denying patent eligibility to cDNA inventions?’ Shelston Intellectual Property (21 August 2017) <http://www.shelstonip.com/news/australian-patent-office-denying-patent-eligibility-cdna-inventions/>
Smith, Sharon, ‘Explaining: Myriad Genetics and BRCA1 Patent Ruling’ Hospital and Healthcare (Web Page, 8 October 2015) <https://www.hospitalhealth.com.au/content/aged-allied-health/article/explainer-myriad-genetics-and-brca1-patent-ruling-867222483#axzz5kkpBdQdG>
United States Patent and Trademark Office, ‘2104 Inventions Patentable – Requirements of 35 U.S.C 101 [R-08.2017]’ Manual of Patent Examining Procedure <https://www.uspto.gov/web/offices/pac/mpep/s2104.html>
‘What is DNA’ U.S. National Library of Medicine (Web Page, 2 April 2019) <https://ghr.nlm.nih.gov/primer/basics/dna>
[1] 569 US 576 (2013).
[3] Robert French, ‘United States Influence on the Australian Legal System’ [2018] UWALawRw 2; (2018) 43(1) University of Western Australia Law Review 11.
[4] ‘History’, Myriad (Web Page) <https://myriad.com/about-myriad/inside-myriad/history/>.
[5] Ibid.
[6] Subhashini Chandrasekharan et al, ‘Do Recent US Supreme Court Rulings on Patenting of Genes and Genetic Diagnostics Affect the Practice of Genetic Screening and Diagnosis in Prenatal and Reproductive Care?’ (2014) 34(10) Prenatal Diagnosis 921, 922.
[7] Association for Molecular Pathology v Myriad Genetics Inc, 133 S Ct 2107.
[8] Sharon Smith, ‘Explaining: Myriad Genetics and BRCA1 Patent Ruling’, Hospital and Healthcare (Web Page, 8 October 2015) <https://www.hospitalhealth.com.au/content/aged-allied-health/article/explainer-myriad-genetics-and-brca1-patent-ruling-867222483#axzz5kkpBdQdG>.
[9] Dan Harrison, ‘Genetic Patents: Grandmother Yvonne D’Arcy Takes on Global Giant Myriad Genetics’, The Sydney Morning Herald (online, 16 June 2015) <https://www.smh.com.au/politics/federal/genetic-patents-grandmother-yvonne-darcy-takes-on-global-giant-myriad-genetics-20150616-ghphvj.html>.
[10] D'Arcy v Myriad Genetics Inc [2015] HCA 35, 3.
[11] ‘What is DNA’, U.S. National Library of Medicine (Web Page, 2 April 2019) <https://ghr.nlm.nih.gov/primer/basics/dna>.
[12] ‘How Many Chromosomes Do People Have?’, U.S. National Library of Medicine (Web Page, 2 April 2019) <https://ghr.nlm.nih.gov/primer/basics/howmanychromosomes>.
[13] Amgen, ‘Identification and Isolation of the Gene of Interest’ (YouTube, 15 August 2016) 00:00:36 – 00:00:45 <https://www.youtube.com/watch?time_continue=38&v=aYhf-FXpoYs>.
[14] ‘cDNA (Complementary DNA)’, HumanGenes.org (Web Page) <http://humangenes.org/cdna-complementary-dna> .
[15] Robert Schwartz, ‘Life After Myriad: The Uncertain Future of Patenting Biomedical Innovation and Personalised Medicine in an International Context’ (2015) 3 Intellectual Property Quarterly 189, 192.
[16] United States Constitution art I § 8 cl 8.
[17] 35 USC § 101.
[18] United States Patent and Trademark Office, ‘2104 Inventions Patentable – Requirements of 35 U.S.C 101 [R-08.2017]’ Manual of Patent Examining Procedure <https://www.uspto.gov/web/offices/pac/mpep/s2104.html>.
[19] Alice Corp v CLS Bank International, 573 US 208 (2014); Mayo Collaborative Services v Prometheus Laboratories Inc, 566 US 66 (2012).
[20] United States Patent and Trademark Office, Manual of Patent Examining Procedure, ‘2106.04(c) The Markedly Different Characteristic Analysis [R-08.2017]’ <https://www.uspto.gov/web/offices/pac/mpep/s2106.html#ch2100_d29a1b_13bc1_b1>.
[21] (Cth).
[22] National Research Development Corporation v Commissioner of Patents [1959] HCA 67, 15 (Dixon CJ, Kitto and Windeyer JJ).
[23] Ibid, 5.
[25] Apotex Pty Ltd v Sanofi-Aventis Australia Pty Ltd [2013] HCA 50, 240 (Crennan J and Kiefel J) (emphasis added).
[26] Schwartz (n 15) 194.
[27] Association for Molecular Pathology v US Patent and Trademark Office, 653 F 3d 1329 (Fed Cir, 2011).
[28] D'Arcy v Myriad Genetics Inc [2015] HCA 35, 90.
[29] Association for Molecular Pathology v Myriad Genetics Inc, 133 S Ct 2107, 2118.
[30] Ibid 2110 (emphasis added).
[31] Ibid 2112.
[32] D'Arcy v Myriad Genetics Inc [2015] HCA 35, 88 (French CJ, Kiefel, Bell and Keane JJ).
[33] Ibid.
[34] Ibid 6 (emphasis added).
[35] Ibid 89.
[36] Schwartz (n 15) 204.
[37] Association for Molecular Pathology v Myriad Genetics Inc, 133 S Ct 2107, 2112.
[38] Brad Sherman, ‘D’Arcy v Myriad Genetics Inc: Patenting Genes in Australia’ [2015] SydLawRw 6; (2015) 37(1) Sydney Law Review 135.
[39] Schwartz (n 15) 204.
[40] National Research Development Corporation v Commissioner of Patents [1959] HCA 67, 8 (Dixon CJ, Kitto and Windeyer JJ).
[41] D'Arcy v Myriad Genetics Inc [2015] HCA 35, 89 (French CJ, Kiefel, Bell and Keane JJ).
[42] Association for Molecular Pathology v Myriad Genetics Inc, 133 S Ct 2107, 2109.
[43] Charles Lawson, ‘Patenting Nucleic Acid Sequences: More Ambiguity from the High Court?’ (2018) 25 Journal of Law and Medicine 741.
[44] Grant Shoebridge, ‘Should the Australian Patent Office Be Denying Patent Eligibility to cDNA Inventions?’, Shelston Intellectual Property (Web Page, 21 August 2017) <http://www.shelstonip.com/news/australian-patent-office-denying-patent-eligibility-cdna-inventions/> .
[45] Association for Molecular Pathology v Myriad Genetics Inc, 133 S Ct 2107.
[46] D'Arcy v Myriad Genetics Inc [2015] HCA 35, 89 (French CJ, Kiefel, Bell and Keane JJ).
[47] Ibid.
[48] Association for Molecular Pathology v Myriad Genetics Inc, 133 S Ct 2107, 2108.
[49] Ibid, 689.
[50] Jack Orford, ‘Association for Molecular Pathology v Myriad Genetics Inc: Are Genes Information or Molecules?’ [2014] SydLawRw 24; (2014) 36(3) Sydney Law Review 557 (emphasis added).
[51] Richard Stern, ‘Association for Molecular Pathology v Myriad Genetics: Sieving the Gene Pool’ (2013) 35(11) European Intellectual Property Review 685, 688.
[52] William Bartlett, ‘D’Arcy v Myriad Genetics Inc [2015] HCA 35: The Plurality’s New Factorial Approach to Patentability Rearticulates the Question Asked in NRCD’ (2015) 24(1) Journal of Law, Information and Science 120.
[53] D'Arcy v Myriad Genetics Inc [2015] HCA 35, 28 (French CJ, Kiefel, Bell and Keane JJ).
[54] Ibid.
[55] Ibid 5.
[56] Ibid 28.
[57] Australian Patent Office, ‘2.9.2.6 Nucleic Acids and Genetic Information’ Manual of Practice and Procedure Manual <http://manuals.ipaustralia.gov.au/patents/Patent_Examiners_Manual.htm#customer_service/Customer_Service_Charter_Timeliness_Guidelines.htm> .
[58] Bartlett (n 52).
[59] D'Arcy v Myriad Genetics Inc [2015] HCA 35, 93 (French CJ, Kiefel, Bell and Keane JJ).
[60] Association for Molecular Pathology v Myriad Genetics Inc, 133 S Ct 2107, 2108.
[61] Michele Wales and Eddie Cartier, ‘The Impact of Myriad on the Future Development and Commercialization of DNA-Based Therapies and Diagnostics’ (2015) 5(12) Cold Spring Harbor Perspectives in Medicine 1, 4.
[62] The Centre for International Economics, ‘Economic Analysis of the Impact of Isolated Human Gene Patents’ (Final Report, May 2013).
[63] Ibid 12.
[64] Carolin Häussler et al, ‘To Be Financed or Not: The Role of Patents for Venture Capital Financing’ (Discussion Paper, Centre of Economic Policy Research, 28 February 2008) 2 cited in Wales and Cartier (n 61) 4.
[65] Wales and Cartier (n 61).
[66] David Hunter, ‘How to Object to Radically New Technologies on the Basis of Justice: The Case of Synthetic Biology’ (2013) 27(8) Bioethics 426.
[67] Anthony Mason, ‘Legislative and Judicial Law-Making: Can We Locate an Identifiable Boundary?’ [2003] AdelLawRw 4; (2003) 24(1) Adelaide Law Review 15.
[68] Schwartz (n 15) 202.
[69] Ibid.
[70] Ibid 191.
[71] Chandrasekharan et al (n 6).
[72] United States Patent and Trademark Office, Manual of Patent Examining Procedure, ‘2106.04(c) The Markedly Different Characteristic Analysis [R-08.2017]’ <https://www.uspto.gov/web/offices/pac/mpep/s2106.html#ch2100_d29a1b_13bc1_b1>.
[73] Wales and Cartier (n 61) 6.
[74] Ibid 3.
[75] Australian Patent Office, ‘2.9.2.6 Nucleic Acids and Genetic Information’ Manual of Practice and Procedure Manual . <http://manuals.ipaustralia.gov.au/patents/Patent_Examiners_Manual.htm#customer_service/Customer_Service_Charter_Timeliness_Guidelines.htm> .
[76] Ibid.
[77] Ibid.
[78] Robert Cook-Deegan and Annie Niehaus, ‘After Myriad: Genetic Testing in the Wake of Recent Supreme Court Decisions about Gene Patents’ (2014) 2(4) Current Genetic Medicine Reports 223.
[79] Emanuela Gambini, ‘In the Aftermath of the “Myriad Case” – Myriad is Denied Preliminary Injunction against Ambry Genetics’ (2014) 5(3) European Journal of Risk Regulation 407, 411.
[80] 774 F 3d 755 (Fed Cir, 2014).
[81] Ibid 758.
[82] Cook-Deegan and Niehaus (n 78).
[83] Gambini (n 79) 411.
[84] Ibid.
[85] In Re Roslin institute (Edinburgh), 750 F 3d 1333 (Fed Cir, 2014).
[86] Diamond v Chakrabarty, [1980] USSC 119; 447 US 303 (1980) cited in Robert Schwartz, ‘Life After Myriad: the uncertain future of patenting biomedical innovation and personalised medicine in an international context’ (2015) 3 Intellectual Property Quarterly 189, 214.
[87] Aria Diagnostic Inc v Sequenom Inc, 726 F 3d 1296 (Fed Cir, 2013).
[88] Schwartz (n 15) 213.
[89] Wales and Cartier (n 61).
[90] Mateo Aboy et al, ‘Myriad’s impact on gene patents’ (2016) 34 National Biotechnology 1119.
[91] Robert Fischer, ‘US Case Analysis: Myriad at the Supreme Court’, Managing Intellectual Property (Web Page, 1 September 2013) <https://www.managingip.com/Article/3261961/US-case-analysis-Myriad-at-the-Supreme-Court.html?ArticleId=3261961>.
[92] Re Arrowhead Research Corporation [2016] APO 70.
[93] Ibid 29 (Delegate Calani).
[94] Apotex Pty Ltd v Sanofi-Aventis Australia Pty Ltd [2013] HCA 50, 143.
[95] Re Arrowhead Research Corporation [2016] APO 70, 31 (Delegate Calani).
[96] Shoebridge (n 44).
[98] Cargill Incorporated v Dow AgroSciences LLC [2016] APO 43, 45.
[99] Shoebridge (n 44).
[101] Ibid 14 (Delegate Wagg).
[102] Tobin Klusty and Richard Weinmeyer, ‘Supreme Court to Myriad Genetics: Synthetic DNA is Patentable but Isolated Genes Are Not’ (2015) 17(9) AMA Journal of Ethics 1, 26.
[103] Wales and Cartier (n 61) 4.
[104] Schwartz (n 15).
[105] Mateo Aboy et al, ‘After Myriad, What Makes a Gene Patent Claim ‘Markedly Different’ from Nature?’ (2017) 35(9) Nature Biotechnology 820.
[106] Dan Burk, ‘Dolly and Alice’ (2015) 2(3) Journal of Law and the Biosciences 606, 608.
[107] Shoebridge (n 44).
[108] Harry Surden, ‘Efficient Uncertainty in Patent Interpretation’ (2011) 68 Washington & Lee Law Review 1737, 1743.
[109] Cat Ferguson, ‘The Future of Biohacking in the Age of Patent Trolls’, The Awl (Web Page, 12 December 2013) <https://www.theawl.com/2013/12/the-future-of-biohacking-in-the-age-of-patent-trolls/>.
[110] Surden (n 108) 1742.
[111] Ibid 1815.
[112] Ibid.
[113] Roger Brownsword and Karen Yeung (eds), Regulating Technologies: Legal Futures, Regulatory Frames and Technological Fixes (Hart Publishing, 2008) cited in Lyria Bennet Moses, ‘How to Think about Law, Regulation and Technology: Problems with ‘Technology as a Regulatory Target’ (2013) 5(1) Law, Innovation and Technology 1, 6.
[114] Chris Dent, ‘The Possibilities of a Regulatory Approach to Answer the Question: Should Genetic Inventions be Patentable?” (2012) 22(1) Journal of Law, Information and Science 16.
AustLII:
Copyright Policy
|
Disclaimers
|
Privacy Policy
|
Feedback
URL: http://www.austlii.edu.au/au/journals/UNSWLawJlStuS/2019/7.html